Green energy is a matter of intensive research. Any time we use fossil fuel to operate our machines and devices, we emit carbon dioxide. Most materials when they are oxidized (burnt) evolve thermal energy which can then be converted into electricity. Hydrogen is one such material which when burnt produces energy and water. The production of hydrogen on an industrial scale is a major problem. Hydrogen occurs combined with carbon as hydrocarbons such as methane and methanol or with oxygen as water. Currently hydrogen is produced from methane by the steam methane reformation (SMR) technique. In doing so for each ton of hydrogen, we generate close to 8 tons of carbon dioxide. Hydrogen can be obtained by splitting water into hydrogen and oxygen by electrolysis, which is a very energy intensive process. However if such energy can be produced from green alternatives (wind and solar, for example), we can get hydrogen with no carbon emission.
Abstract: A hydride, produced from water and a metal, is reacted with an oxide ore to yield a metal and hydrogen with significant profit.
Background: Hydrogen, bound in water, is an energy carrier; it can be transported everywhere, and it yields energy when oxidized. When hydrogen and oxygen combine they release energy which can be used to perform all kinds of tasks. To use hydrogen for transportation (e.g. to power an automobile), we need it in an easily storable form. A kilogram of hydrogen can produce energy equivalent to a gallon of gasoline energy Storing hydrogen is difficult. It is the smallest atom in the periodic table and it tends to diffuse out of most containers. We can liquify it, but that’s expensive. An ideal storage method would be a solid that contains hydrogen, and which releases hydrogen on demand without requiring any substantial energy input. Magnesium hydride (MgH2) is such a solid.
of tasks. To use hydrogen for transportation (e.g. to power an automobile), we need it in an easily storable form. A kilogram of hydrogen can produce energy equivalent to a gallon of gasoline energy Storing hydrogen is difficult. It is the smallest atom in the periodic table and it tends to diffuse out of most containers. We can liquify it, but that’s expensive. An ideal storage method would be a solid that contains hydrogen, and which releases hydrogen on demand without requiring any substantial energy input. Magnesium hydride (MgH2) is such a solid.
Direct synthesis of MgH2 from the elements Mg and H2: The problem is that neither hydrogen nor magnesium occur freely in nature. Mg occurs principally in rocks as a carbonate (all the mountains in Italy with dolomites) or as magnesium chloride in the sea-water. The starting point of recovering magnesium from natural resources is the oxide MgO. MgCl2 is the main precursor to metallic magnesium. The conversion is done by electrolysis: MgCl2 → Mg + Cl2. Electrolysis is also the method to get Mg from MgO; the process is called the solid-oxide-membrane (SOM) process (Materials Transactions,36B,463 (2005) and purports to bring the price to as low as $1.50 for a kilogram of Mg. The industrial method of steam methane reformation process gets hydrogen for as little as $1.50. per kilogram. But this comes with a carbon penalty of 8.2 kg of CO2 per kg of hydrogen. There is a method that uses water and Mg to get hydrogen [US Patent#789251], without carbon emission.
We need to consider the energy for electrolysis. For the SOM process we need 12 kWh per Kg of Mg. Can we get this from alternate forms of energy (hydro, nuclear, solar, wind)? It will be ideal if some or all energy could come from the renewable energy sources, but if not, we could still benefit from the proposal outlined below. From what is summarized above, we note that H2 may cost between $1.50 to $3.0 per kilogram and Mg price may be as low as $1.50/kg. With these prices we can replace all fossil fuel uses with hydrogen giving us energy and simply water.
The invented process: The prices indicated above should be possible soon. Hydrogen from methane reformation is cheap but it is not good for the environment. To figure out a method to reduce hydrogen prices, it is proposed that we coproduce hydrogen with something that sells for a high enough price and compensates for the high price of hydrogen (electrolysis). The invented process involves reacting the magnesium hydride with one metal oxide (ore), whereby the oxide is reduced to metal and hydrogen is released e.g. tin oxide is added to magnesium hydride which gives us magnesium oxide, tin and hydrogen (MgH2 + .5 SnO2 = MgO +.5 Sn + H2). The price of half a mole of tin (in kg) is $1305, which means that production of one mole of hydrogen gives a profit close to $1300.0. This calculation considers the materials only.
Patented process: Contact surendraasaxena8@gmail.com

Fig.3. SnO2 and MgH2. Tin oxide (cassiterite) is reduced over large T-X areas; the gas is all hydrogen.
Carbon sequestration by splitting CO2 into carbon and oxygen
The accumulation of CO2 in the atmosphere is harmful to the environment. Carbon dioxide is a very stable gas and the carbon-oxygen bond is not easily broken. It is possible to lock it in a carbonate but then we need an oxide such as MgO or CaO to form a carbonate. These oxides do not occur freely in nature and they need to be produced by the dissociation of the naturally occurring dolomite or other carbonates. Production of a mole of oxide from a carbonate generates a mole of CO2 (for example for the production of 56 grams of CaO results in 44 grams of CO2). Alternative method of sequestering CO2 is to bury the gas underground in various geologic structures but that is costly and unsafe.
The elements Mg, Ca, Si and Al react with CO2 and other oxides and split it into the constituent elements. This chemical property of these elements is not often used in industry because it is considered that production of the elements from the oxide or chloride is an expensive process. Of late progress has been made in the field of electrolysis to reduce the temperature and the carbon footprint of the process, for example, to reduce MgO or MgCl2 to Mg metal. It is estimated that electrolytic reduction can be achieved at the cost of under $2 per kilogram of Mg [1]. The carbon footprint, which is a whopping 11.8 kg per kilogram of Mg, can be reduced to almost nothing by use of alternate energy [2].
The calculations have been performed with the databases available in FactSage 7.2 but any database (such as JANAF Tables or NIST) may be used. We also explore the economic consequences of adopting such chemical methods (price data on elements in [3]); if the economy of the process does not support it, not much will happen in the industrial world.
In summary, Carbon dioxide may be split into carbon and oxygen by reacting with certain elements (Mg ,Ca, Al or Si) which are oxidized and the carbon is released as graphite or forms a carbide. We take the advantage of the exothermic process and reduce simultaneously an oxide (such as SnO2) to its elements or form a carbide. The cost of using the element (Mg, Al, Ca or Si) is more than recovered by selling the metal or the carbide.
Patented process: Contact surendrasaxena8@gmail.com
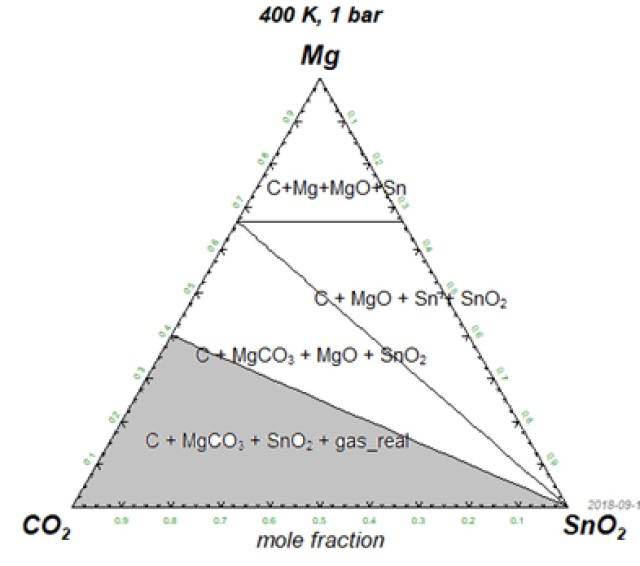
Fig.1a. The pseudo ternary system CO2-SnO2-Mg. The grey area shows where CO2 is stable. In other areas CO2 breaks down and forms graphite, oxides and tin.

Fig. A solar-hydro house with a hydride container of 0.4×0.4×0.4 meters, which accommodates enough hydride for a week’s worth of typical energy use. A portion of this hydride is fed into a smaller container, which is then heated (using solar energy or another source), releasing hydrogen for daily energy consumption. This hydrogen can be fed to a fuel cell for later use, including at night. Hydrogen also has many other household uses.
